A sigma bond is when two S or P orbitals overlap horizontally. A pi bond is when two P orbitals, that are parallel, overlap. Sigma bonds are stronger, because they have atomic orbitals that have been hybernized. Also, the way the bonds are formed is an important factor; pi bonds allow a space in the middle of the bond, while sigma bonds do not.( See pictures below for a better understanding). The overlap of the orbitals is larger in sigma bonds, so they are harder to split.
 |
| Sigma Bond w/ S orbitals |
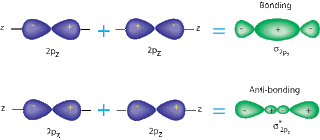 |
| Sigma Bond w/ P orbitals |
 |
| Pi Bond w/ P orbital |
31) This question is from the chpt. 8 review packet, it is number 17. "Explain how a decrease in the vapor pressure of a solution results in an increase in its boiling point."
-Vapor pressure lowers when a solute is added.
-Fewer solute particles will escape to the liquid's surface.
-The solvent molecules that surround the particles dissolve in the solution, which means that fewer solvent molecules are available to escape to the surface.
Emily, your blog looks great and is very imformative however I have a slight suggestion to add onto number 31. You didn't exactly explain WHY there is an increase in boiling point. It might be helpful to say that vapor pressure pushes up against the atmospheric pressure when the vapor pressure=atmospheric pressure. If you reduce the vapor pressure, it is harder for the molecules to escape therfore there will be an incrase in boiling point.
ReplyDeleteThe information is correct, the picture also helps explain and goes well with your description.
ReplyDelete