A sigma bond is when two S or P orbitals overlap horizontally. A pi bond is when two P orbitals, that are parallel, overlap. Sigma bonds are stronger, because they have atomic orbitals that have been hybernized. Also, the way the bonds are formed is an important factor; pi bonds allow a space in the middle of the bond, while sigma bonds do not.( See pictures below for a better understanding). The overlap of the orbitals is larger in sigma bonds, so they are harder to split.
 |
| Sigma Bond w/ S orbitals |
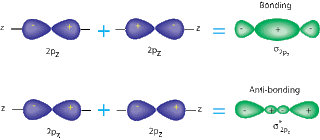 |
| Sigma Bond w/ P orbitals |
 |
| Pi Bond w/ P orbital |
31) This question is from the chpt. 8 review packet, it is number 17. "Explain how a decrease in the vapor pressure of a solution results in an increase in its boiling point."
-Vapor pressure lowers when a solute is added.
-Fewer solute particles will escape to the liquid's surface.
-The solvent molecules that surround the particles dissolve in the solution, which means that fewer solvent molecules are available to escape to the surface.